Kaori Morimoto1, Abigail Calkins1, Jacob Fletcher DO1, Anita Nelson MD1
PNWMSRJ. Published online November 2nd, 2021.Abstract:
Background: The prevalence of diabetes mellitus (DM) in pregnant women is increasing in the US. Diabetes is associated with a higher risk of pregnancy complications such as fetal macrosomia and cesarean sections. Multiple studies have investigated the efficacy of continuous glucose monitor (CGM) use in reducing these complications.
Methods: A systematic review of primary articles was conducted using nine medical journal search engines. A total of 2,028 cases were gathered from 17 studies between the years 2007 and 2020. Data regarding secondary endpoints, including neonatal outcomes, were also collected. Results were compared using relative risk and Pearson’s Chi-square test.
Results: The relative risk for cesarean delivery in the CGM group was 0.96 (95% CI: 0.67-1.24) compared to the control, and the relative risk for fetal macrosomia in the CGM group was 0.93 (95% CI: 0.55-1.30). Interestingly, women diagnosed with gestational DM and used CGMs during pregnancy had similar rates of LGA neonates across China, Malaysia, and European countries. However, the rate of cesarean section significantly differed in China and Malaysia compared with European countries.
Conclusion: CGM use was not associated with a reduction in diabetes-related pregnancy complications. The cesarean section rate was significantly different depending on the region, which may indicate a difference in clinical practice among those regions.
Relevance: To the best of our knowledge, this meta-analysis utilized a larger sample size than any other published literature to explore the impact of CGM use on pregnancy complications.
Introduction:
In the US, diabetes during pregnancy is on the rise.[1,2] In 2016, 0.9% of live births were to mothers with preexisting DM (type I and II). 6% of live births involved gestational diabetes.[3]
Maternal diabetes is linked to various adverse pregnancy outcomes, including increased preeclampsia, preterm delivery, and cesarean section.[4] Infants of mothers with preexisting DM are reported to have a greater risk of perinatal mortality, congenital anomaly, premature birth, being born large for gestational age (LGA), and neonatal hypoglycemia.[5,6] Preexisting diabetic mothers have an increased incidence of elective and emergency cesarean sections.7 Furthermore, preexisting diabetes is linked to perinatal mortality four times higher than non-diabetic pregnancy mothers.[8] One of the most crucial factors in diabetes management is optimal glucose control. Several studies show that poor glycemic control, resulting in elevated HbA1C, during early pregnancy is associated with an increased risk of congenital anomalies, stillbirth, and miscarriage.[8-10] Also, elevated HbA1C during the third trimester was associated with prematurity, low birth rate, and fetal macrosomia.11 Fetal macrosomia is a common indication for the cesarean section, which has a maternal risk of infections, organ injuries, hysterectomy, chronic pain, and so on.11 When compared to vaginal deliveries, neonates delivered via cesarean section had significantly higher respiratory morbidity.[12] For healthy fetal growth, optimal glycemic control is crucial; however, achieving it remains challenging for many mothers with preexisting diabetes.[13] CGM was developed in 1999 to enable patients to continuously monitor their blood glucose level throughout the day by detecting the interstitial fluid glucose level through a small device inserted under the skin.14 Depending on the brand, the sensor can be worn for 5-90 days.[14,15] When CGM is worn, finger prick monitoring is only required for occasional calibration. Results can either be read through an at-home monitor or by a provider. Some CGM devices have alarms for when patients are either hyperglycemic or hypoglycemic.14 While CGM is more expensive than finger prick monitoring, CGM has high patient satisfaction.[16] Multiple studies have shown that incidence of LGA, cesarean sections, and preeclampsia did not improve with use of CGM during pregnacy.[17,18] One study found that CGM is a worthwhile pursuit for mothers with type 1 diabetes because of lower HbA1C (-0.19%) and increased time in target glucose range when compared to mothers using finger prick monitoring.[19] Another benefit was that infants of mothers with CGM required fewer treatments for hypoglycemia.[18,19] This review aims to analyze the up-to-date literature to further investigate the clinical efficacy of CGM use for the management of diabetes during pregnancy. Additionally, the regional pregnancy outcomes among the CGM use group were analyzed to understand the impact.Methods:
Data about the pregnancy outcomes of mothers with type I, type II, or gestational DM who have used a CGM during pregnancy was obtained through the following nine major medical literature search engines: Pubmed, Google Scholar, Embase, PMC, Europe PMC, CINAHL, Cochrane Library, MeSHMEDLINE, and ERIC. The articles published from inception to May 10th, 2020, were included.
Exclusion criteria were the followings: nonpregnant patients, no history of type I or type II DM, no diagnosis of gestational DM during the current pregnancy, blood glucose monitoring with methods other than a CGM, or research that did not include data regarding fetus LGA, or rate of cesarean delivery. After the selection, 17 articles were included. The selection process is summarized in image 1.
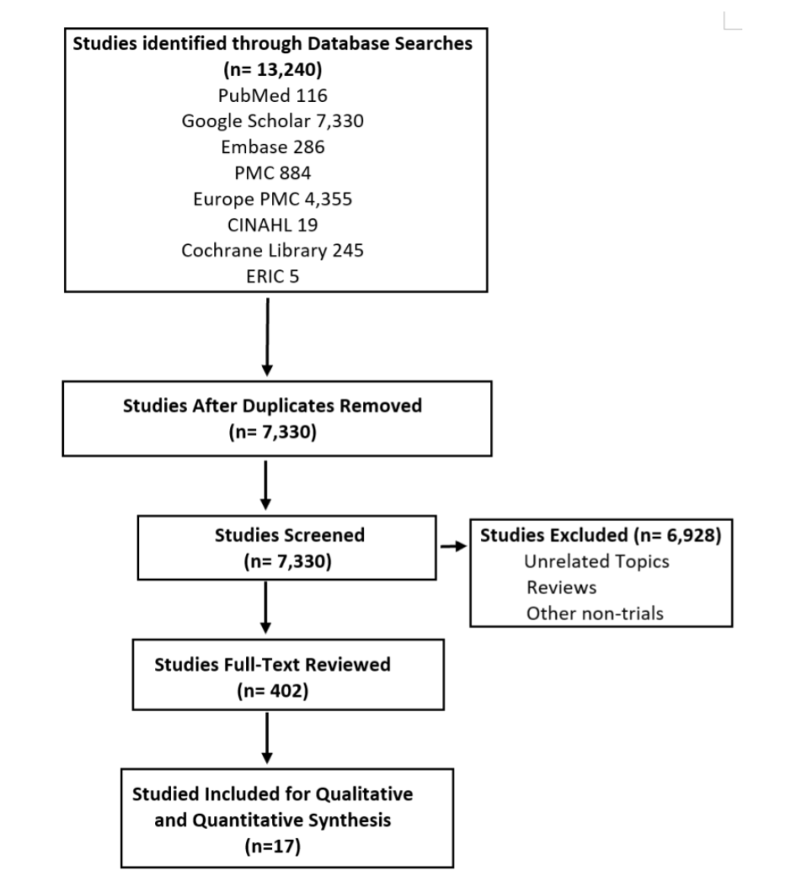
For statistical analysis, the SAS University program was used to calculate the relative risk and Pearson’s Chi-square test. The relative risk analysis was conducted to determine the correlation of having specific pregnancy outcomes such as cesarean delivery, LGA, neonatal hypoglycemia, prematurity, and preeclampsia in the CGM use group in contrast with the control group. Similarly, the Chi-square test was used for the analysis of four different correlations: 1) CGM use (user vs. non-user) and pregnancy complications, 2) region in the world (Asia vs. Europe, Asia vs. the Middle East vs. Europe), and cesarean section frequency in mothers who used CGM, 3) region in the world (Asia vs. Europe) and LGA frequency in mothers who used CGM, and 4) type of diabetes (Type I vs. GDM) and pregnancy complication frequency.
Results:
This review comprises 17 studies with 2,028 patients from Asia (East, Southeast, and Middle), US, Canada, and Europe, diagnosed with type I, type II, or gestational diabetes. Among these, 1,292 cases used CGM for a certain period during pregnancy, and 736 patients used the finger-prick method exclusively to monitor their blood glucose throughout pregnancy. CGMs were primarily used as an educational tool to improve glycemic control during pregnancy, and the pregnancy outcomes were measured.
10 studies were from Europe,[17, 19-27] 4 studies were from the US,[19, 28-30] 2 studies were from China,[31, 32] one study was from Malaysia,[33] and one study was from Saudi Arabia.[34] One of those studies was conducted in the US, Canada, and European countries.[19] The primary outcomes of this study are summarized in Table 1.
The CGM group consists of women who used CGMs during pregnancy and were diagnosed with either type I, II, or gestational DM. The Control group consists of women who used finger prick exclusively for monitoring their blood glucose level throughout pregnancy. Some studies did not specify the number of patients with each type of diabetes. 379 patients who used CGM without specification of their diabetes type (type I vs. type II vs. gestational) are included in the CGM group. The chi-square results and relative risk (RR) for pregnancy-related complications among the CGM user vs. non-user are also summarized in Table 1. None of those have statistically significant outcomes.
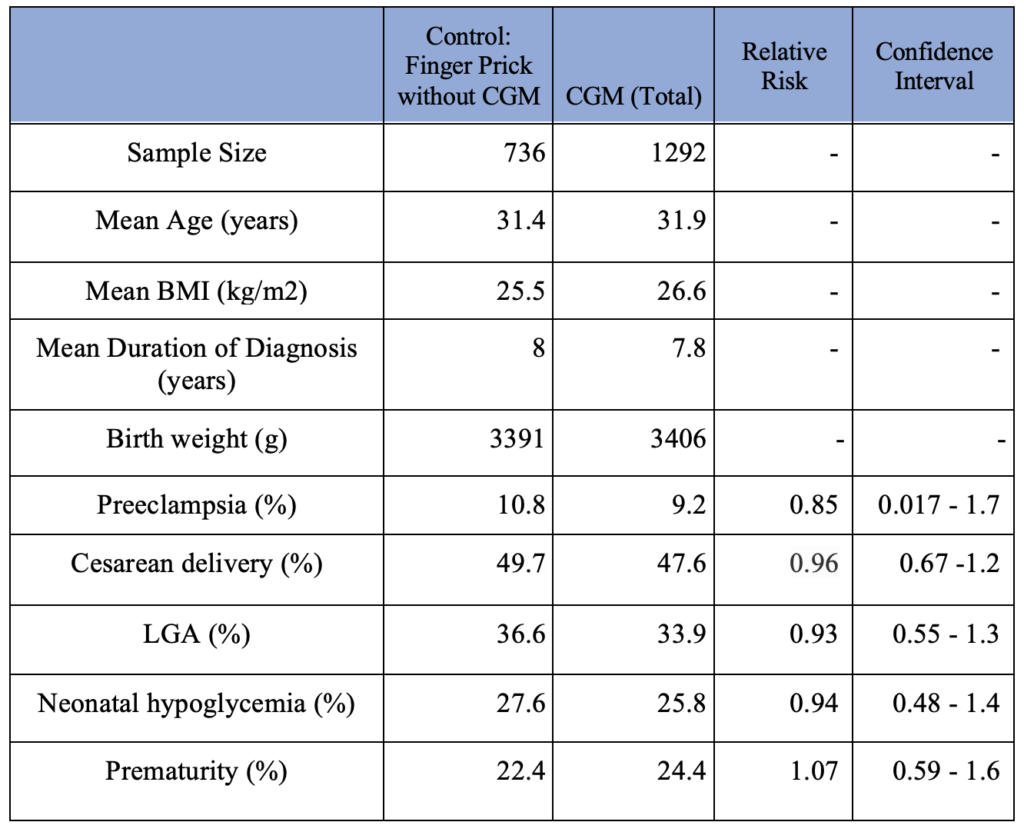
Table 1. Pregnancy Complication Outcomes in Patients (CGM use vs. non-CGM use)
The secondary outcomes are summarized in Table 2. The type I DM group consists of women diagnosed with type I diabetes and used CGM during pregnancy. The gestational DM group consists of women diagnosed with gestational diabetes and used CGM during pregnancy. For calculating the duration of diabetes, 0.4 years was used for the duration of gestational diabetes uniformly as gestational diabetes is routinely screened between 24th and 28th gestational weeks. Type II DM patients were not included due to a small sample size. Additionally, patients with unspecified types of DM were not included.
The type of diabetes (type I vs. gestational) among mothers who used CGM showed a strong correlation with the frequency of pregnancy-related complications such as preeclampsia, cesarean delivery, LGA, neonatal hypoglycemia, and prematurity. In other words, CGM use did not close the gap between the pregnancy-related complication rate between type I GM and gestational GM patients. The results are summarized in Table 2.
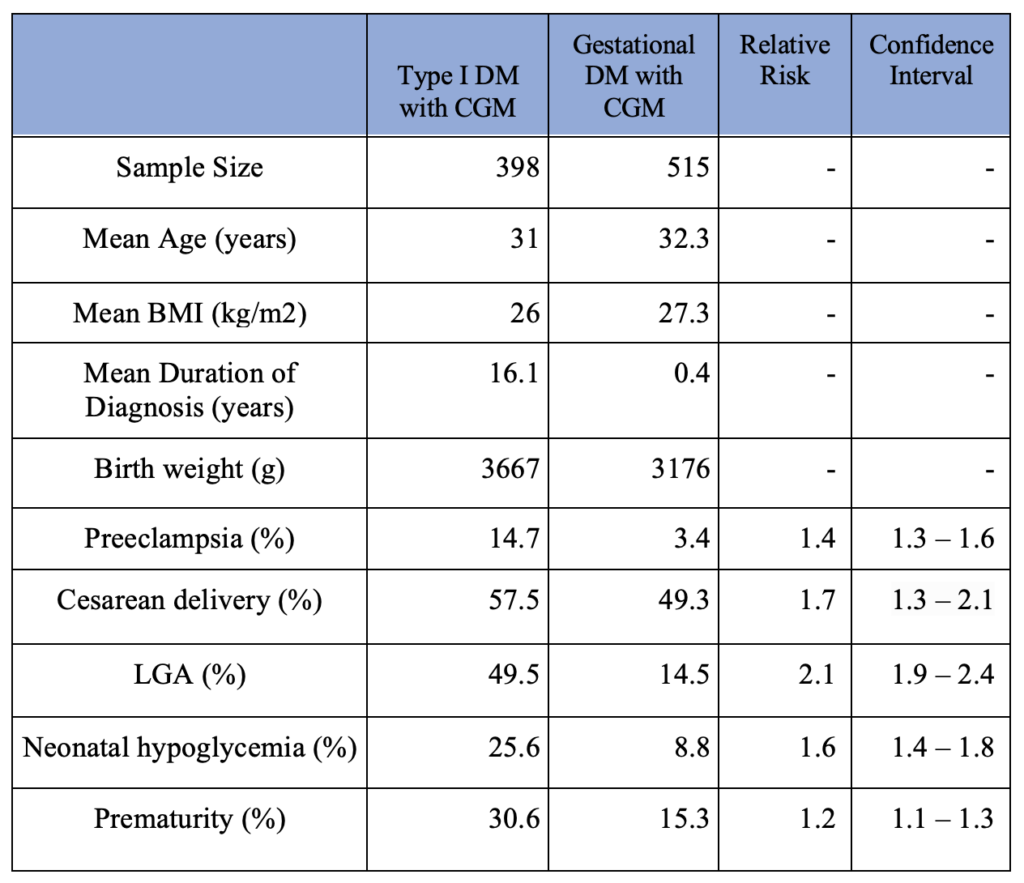
Table 2. Pregnancy Complication Outcomes in CGM use Patients (Type I DM vs. Gestational DM)
The demographics of the patients who used CGM during pregnancy based on the regions in the world are summarized in Table 3. The East/Southeast Asia group consists of women from China and Malaysia. The Middle East Asia group consists of women from Saudi Arabia. The European group consists of women from Belgium, Denmark, England, Finland, France, Ireland, Italy, Netherland, Scotland, Spain, and Sweden.
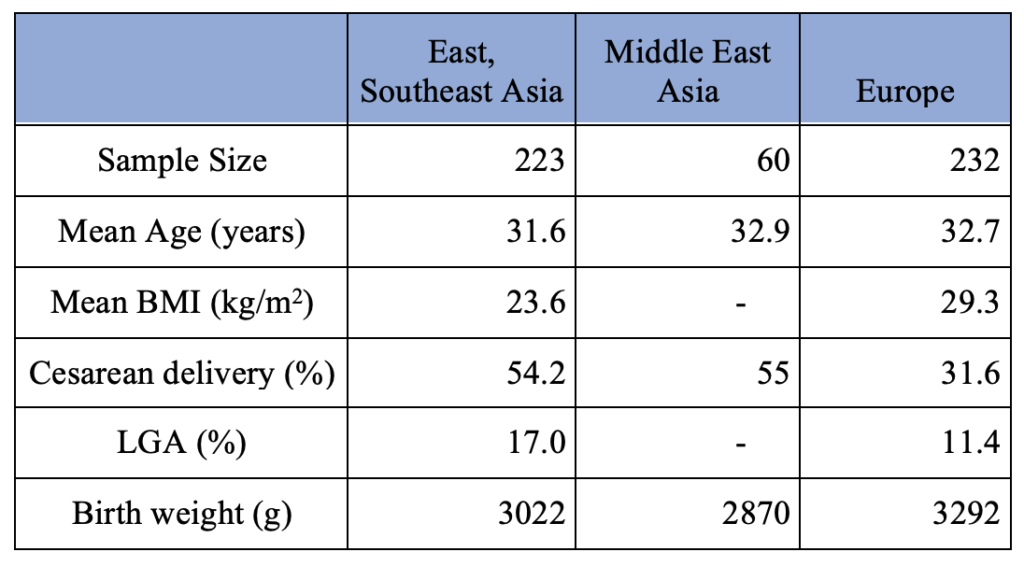
Table 3. Pregnancy Outcome of Gestational Diabetes Mothers with Intermittent CGM Use per Region
Chi-square test results are summarized in Table 4. A P-value of 0.05 was considered statistically significant. The Chi-square test revealed a statistically significant association between the region in the world (East and Southeast Asia vs. Middle East Asia vs. Europe) and the rate of cesarean section in mothers with gestational diabetes who used CGM during their pregnancy. The Chi-square value was 12.82 with a degree of freedom (df) of 2, and the p-value was 0.001644. Also, the statistically significant difference in the cesarean section rate between East/Southeast Asia and Europe was found based on the Chi-square value of 11.95, df of 1, and the p-value of 0.000548. However, the association between the region (East and Southeast Asia vs. Europe) and the rate of LGA in mothers with gestational diabetes was statistically insignificant based on the Chi-square value of 2.576 with df of 1 and the p-value of 0.1085. These results reveal that even though the rate of LGA among mothers diagnosed with gestational diabetes and used CGM in Asia and Europe was comparable, the cesarean section rate was not.

Table 4. Gestational DM Mothers Pregnancy Complication vs. Region in the world
Discussion:
Our primary outcome agrees with previous research that the periodical CGM use during pregnancy did not have a significant impact on the pregnancy outcomes compared with the group who used the finger-prick method to monitor their blood glucose level.[18, 35] While a randomized control trial conducted by Voormolen et al. included all three types of diabetes mellitus, Murphy et al., Secher et al., and Law et al. only focused on type I and II diabetes.[20, 21, 23, 24] Two small studies conducted by Taslimi et al. and Dalfra et al. included both type I diabetes and gestational diabetes.[26,30] In contrast, multiple studies limited the study population to women diagnosed with one specific type of diabetes mellitus. The studies conducted by Feig et al., Polsky et al., Kristensen et al., and Mulla et al. focused on type I diabetes.[17,19,28,29] Whereas Franck et al., Kestila et al., Yu et al., Alfadhli et al., Paramasivam et al., Law et al., and Wei et al., solely focused on the women with gestational diabetes.[22,25,27,31,32,33,34] Regardless of these differences, all studies conclude that CGM use did not improve the glycemic control which translated to no significant difference in pregnancy outcomes.
One possible explanation for the non-significant effect of CGM use during pregnancy compared to finger prick would be CGMs compliance and frequency of use throughout pregnancy. In the DIAMOND randomized clinical trial, type I diabetic patients used CGMs for 24 consecutive weeks, and the HbA1C level, blood glucose level target in range time, and the duration of hypoglycemia in the CGM group decreased significantly.36 In contrast, in many studies included in this review, the women were required to wear a CGM 5 to 7 days every 4 to 6 weeks.[20,23,24,26,29,32,33] Other studies required women to wear CGMs once during pregnancy for two days to 1 month. [22,31,34]
In the CONCEPTT trial, 325 women with Type I DM were enrolled and randomly assigned to the CGM group with finger prick monitoring or the control group with the finger prick monitoring only. The study group wore the CGM throughout the pregnancy and the neonatal outcomes showed statistically significant improvement with a lower rate of LGA, NICU admission, and neonatal hypoglycemic episodes. On average, the CGM group had one shorter day of hospital stay.[19] In the sub-analysis of the CONCEPTT trial, the progressive improvement in the time in glucose target range among the CGM group was found as the pregnancy progressed: 7.7 % during the first trimester, 10.2% during the second trimester, and 35.5% during the third trimester. This would explain why the study found lower neonatal complications associated with poor glucose control in later trimesters such as LGA.44 This suggests that wearing CGM throughout pregnancy would be overall beneficial for this population.
Our secondary outcomes highlighted some important facts to consider for future studies measuring pregnancy outcomes. The Chi-square tests for women diagnosed with gestational DM and used CGMs during pregnancy showed similar rates of LGA neonates across East/Southeast Asia and European countries. However, the rate of cesarean section was significantly higher in Asia compared with European countries. This may indicate a difference in clinical practice by region and country. In fact, in a study of 121 countries, the cesarean section rate in Asia was 15.1%, while that of Europe was 13.8%, which is higher than the world average of 12.4%.[37]
This difference in the threshold for cesarean section based on the region in the world is interesting and almost contra-intuitive considering that higher BMI is one of the risk factors for cesarean section. In our study, the average BMI of East/Southeast Asian mothers was 23.6 kg/m2, which is within the normal range, while European mothers were 29.3 kg/m2, which falls in the overweight category.38 Multiple large studies, including the HAPO study, indicate a strong association between the increase in the rate of cesarean section and the maternal BMI.[39,40] This suggests that the variability in clinical practice may have a stronger impact in the rate of cesarean section than maternal BMI.
Some of the confounding variables of this study include the use of different brands of CGMs, the difference in duration and the timing of the CGM use, the variation in viewing options of the CGM reports, women having different types of diabetes mellitus, and non-standardized care for each patient.
The types of continuous CGMs used in each study varied. Some of the CGMs used in the studies included Guardian Real-time CGM, Minimed Medtronic, Freestyle Libre, and iPro2 Professional CGM.[17, 24, 31, 32] Additionally, the duration and the timing of the CGM use were not identical across the studies.
As mentioned above, most of the studies included had patients use CGMs for less than a week for multiple non-consecutive weeks.[20, 23, 24, 26, 29, 32, 33] Some studies had the mothers wear CGMs once for a variety of periods.[22, 31, 34] For the most accurate results, having an identical period and duration of CGM use across participants would be beneficial.
While most studies allowed patients to view their blood glucose level in real-time, some studies only had glucose level reviewing during office visits.17 CGM is advantageous because it gives patients immediate feedback about the effect of diet, medications, exercise, and insulin injections on their blood glucose levels.41 Thus, having different modes of viewing may affect the study outcomes indefinitely.
In our study, pregnant women with all types of diabetes mellitus were included similarly to a previous study by Voormolen et al.[20] While this approach increases statistical power, including all types of maternal diabetes is a confounding variable. As previous studies showed, preexisting maternal type I or type II DM is strongly associated with pregnancy-related complications such as premature birth, LGA, and neonatal hypoglycemia compared to no maternal DM or gestational DM.[5, 6] Therefore, the impact of those should be analyzed in the future studies.
The care and appointment time for each patient were not standardized within each study and across the studies. The standard treatments for diabetes are lifestyle change (diet and exercise), medications such as metformin and glyburide, and insulin therapy.[42]
Lifestyle medicine is an emerging field, and its benefits on chronic conditions are promising. Mothers of gestational diabetes who received lifestyle medicine intervention had lower rates of postnatal depression and neonates large for gestational age.[43] One study, included in this systematic review, reported an outstandingly lower LGA rate compared to other studies. While the rate of LGA for other studies fell in the range of 13% to 63%, with the median value of 34.8%, the study by Law et al. reported only 9.2% of neonates classified as LGA. Interestingly, their primary intervention was lifestyle modification. Therefore, providing standardized care would be beneficial to assess the true effectiveness of CGMs in future studies.[22]
Conclusion:
This study included two thousand twenty-eight pregnant women with a history of type I, type II, and gestational DM from 17 studies. The evidence suggests that intermittent CGM use has no significant impact on improving pregnancy outcomes, including the rates of preeclampsia, cesarean section, large for gestational age fetus, prematurity, and neonatal hypoglycemia. Secondary findings suggest that country/region may affect the rate of cesarean section, but not LGA. Additionally, the rate of cesarean section and LGA fetuses in type I diabetes mothers with CGM use were significantly higher than those of gestational diabetes mothers. Further study is indicated to investigate the efficacy of continuous CGM use with various viewing options for the diabetes-associated complication risk reduction in pregnant patients.
Acknowledgements:
We appreciate Dr. Anita Nelson for her help and advice on the paper.
References
- Bardenheier BH, Imperatore G, Devlin HM, Kim SY, Cho P, Geiss LS. Trends in pre-pregnancy diabetes among deliveries in 19 U.S. states, 2000-2010. Am J Prev Med. 2015;48(2):154‐161. doi:10.1016/j.amepre.2014.08.031
- Bardenheier BH, Imperatore G, Gilboa SM, et al. Trends in Gestational Diabetes Among Hospital Deliveries in 19 U.S. States, 2000-2010. Am J Prev Med. 2015;49(1):12‐19. doi:10.1016/j.amepre.2015.01.026
- Deputy NP, Kim SY, Conrey EJ, Bullard KM. Prevalence and Changes in Preexisting Diabetes and Gestational Diabetes Among Women Who Had a Live Birth – United States, 2012-2016. MMWR Morb Mortal Wkly Rep. 2018;67(43):1201‐1207. Published 2018 Nov 2. doi:10.15585/mmwr.mm6743a2
- Knight KM, Thornburg LL, Pressman EK. Pregnancy outcomes in type 2 diabetic patients as compared with type 1 diabetic patients and nondiabetic controls. J Reprod Med. 2012;57(9-10):397‐404.
- Yang J, Cummings EA, Oʼconnell C, Jangaard K. Fetal and Neonatal Outcomes of Diabetic Pregnancies. Obstetrics & Gynecology. 2006;108(3, Part 1):644-650. doi:10.1097/01.aog.0000231688.08263.47.
- Cordero L, Treuer SH, Landon MB, Gabbe SG. Management of infants of diabetic mothers. Arch Pediatr Adolesc Med. 1998;152(3):249‐254. doi:10.1001/archpedi.152.3.249
- Mackin ST, Nelson SM, Kerssens JJ, et al. Diabetes and pregnancy: national trends over a 15 year period. Diabetologia. 2018;61(5):1081‐1088. doi:10.1007/s00125-017-4529-3
- Macintosh MC, Fleming KM, Bailey JA, et al. Perinatal mortality and congenital anomalies in babies of women with type 1 or type 2 diabetes in England, Wales, and Northern Ireland: population based study. BMJ. 2006;333(7560):177. doi:10.1136/bmj.38856.692986.AE
- Suhonen L, Hiilesmaa V, Teramo K. Glycaemic control during early pregnancy and fetal malformations in women with type I diabetes mellitus. Diabetologia. 2000;43(1):79‐82. doi:10.1007/s001250050010
- Mills JL, Simpson JL, Driscoll SG, et al. Incidence of spontaneous abortion among normal women and insulin-dependent diabetic women whose pregnancies were identified within 21 days of conception. N Engl J Med. 1988;319(25):1617‐1623. doi:10.1056/NEJM198812223192501
- Gonzalez-Gonzalez NL, Ramirez O, Mozas J, et al. Factors influencing pregnancy outcome in women with type 2 versus type 1 diabetes mellitus. Acta Obstet Gynecol Scand. 2008;87(1):43‐49. doi:10.1080/00016340701778732
- Mylonas I, Friese K. Indications for and Risks of Elective Cesarean Section. Deutsches Aerzteblatt Online. 2015. doi:10.3238/arztebl.2015.0489
- Murphy HR, Bell R, Cartwright C, et al. Improved pregnancy outcomes in women with type 1 and type 2 diabetes but substantial clinic-to-clinic variations: a prospective nationwide study. Diabetologia. 2017;60(9):1668-1677. doi:10.1007/s00125-017-4314-3.
- Olczuk D, Priefer R. A history of continuous glucose monitors (CGMs) in self-monitoring of diabetes mellitus. Diabetes & Metabolic Syndrome: Clinical Research & Reviews. 2018;12(2):181-187. doi:10.1016/j.dsx.2017.09.005.
- Eversense Continuous Glucose Monitoring: Long-term Continuous Glucose Monitor. Eversense Continuous Glucose Monitoring | Long-term Continuous Glucose Monitor. https://www.eversensediabetes.com/. Accessed May 24, 2020.
- Health Quality Ontario. Continuous Monitoring of Glucose for Type 1 Diabetes: A Health Technology Assessment. Ont Health Technol Assess Ser. 2018;18(2):1‐160. Published 2018 Feb 21.
- Kristensen, et al. Continuous glucose monitoring in pregnant women with type 1 diabetes: an observational cohort study of 186 pregnancies. Diabetologia. 2019;62:1143–1153. doi: 10.1007/s00125-019-4850-0.
- Jones LV, Ray A, Moy FM, Buckley BS. Techniques of monitoring blood glucose during pregnancy for women with preexisting diabetes. Cochrane Database of Systematic Reviews. 2019;5(5):CD009613. Published 2019 May 23. doi:10.1002/14651858.CD009613.pub4
- Feig DS, Donovan LE, Corcoy R, Murphy KE, Amiel SA, Hunt KF, et al. Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multicentre international randomised controlled trial. Lancet. 2017;390(10110):2347‐2359. doi:10.1016/S0140-6736(17)32400-5
- Voormolen DN, DeVries JH, Sanson RM, et al. Continuous glucose monitoring during diabetic pregnancy (GlucoMOMS): A multicentre randomized controlled trial. Diabetes Obesity and Metabolism. March 2018:1894-1902. https://doi-org.proxy.westernu.edu/10.1111/dom.13310. Accessed May 14, 2020.
- Law GR, Ellison GT, Secher AL, et al. Analysis of continuous glucose monitoring in pregnant women with diabetes: distinct temporal patterns of glucose associated with large-for-gestational-age infants. Diabetes Care. 2015;38:1319–1325
- Law GR, Gilthorpe MS, Secher AL, et al. Translating HbA1c measurements into estimated average glucose values in pregnant women with diabetes. Diabetologia. 2017;60(4):618–624. doi: 10.1007/s00125-017-4205-7.
- Murphy HR, Rayman G, Lewis K, et al. Effectiveness of continuous glucose monitoring in pregnant women with diabetes: randomised clinical trial. Bmj. 2008;337(sep25 2). doi:10.1136/bmj.a1680
- Secher AL, Ringholm L, Andersen HU, Damm P, Mathiesen ER. The Effect of Real-Time Continuous Glucose Monitoring in Pregnant Women With Diabetes: A randomized controlled trial. Diabetes Care. 2013;36(7):1877-1883. doi:10.2337/dc12-2360
- Kestile K, Ekblad U, Ronnemaa T. Continuous glucose monitoring versus self-monitoring of blood glucose in the treatment of gestational diabetes mellitus. Diabetes Research and Clinical Practice. 2007;77(2):174-179. https://doi.org/10.1016/j.diabres.2006.12.012.
- Dalfrà M, et al. Glucose Variability in Diabetic Pregnancy. Diabetes Technology & Therapeutics. 2011;13(8):853-859.
- Franck M, Philips JC, Emonts P, Radermecker RP. Contribution of continuous glucose measurement in the management of gestational diabetes: a pilot study. Rev Med Liege. 2017 Jun;72(6):295-300
- Polsky S, Garcetti R, Pyle L, Joshee P, Demmitt JK, Snell-Bergeon JK. Continuous Glucose Monitor Use With Remote Monitoring Reduces Fear of Hypoglycemia in Pregnant Women With Type 1 Diabetes: A Pilot Study. J Diabetes Sci Technol. 2019:1932296819890864
- Mulla B, et al. Continuous Glucose Monitoring, Glycemic Variability, and Excessive Fetal Growth in Pregnancies Complicated by Type 1 Diabetes. Diabetes Technology & Therapeutics 2018;20(6), 413-419.
- Taslimi MM, Navabi K, Acosta R, Helmer A, El-Sayed YY. Concealed maternal blood glucose excursions correlate with birth weight centile. J Diabetes Sci Technol. 2008;2:456-460.
- Wei Q, Sun Z, Yang Y, Yu H, Ding H, Wang S. Effect of a CGMS and SMBG on Maternal and Neonatal Outcomes in Gestational Diabetes Mellitus: a Randomized Controlled Trial. Scientific Reports. 2016;6(1). doi:10.1038/srep19920.
- Yu F, Lv L, Liang Z, et al. Continuous Glucose Monitoring Effects on Maternal Glycemic Control and Pregnancy Outcomes in Patients With Gestational Diabetes Mellitus: A Prospective Cohort Study. The Journal of Clinical Endocrinology & Metabolism. 2014;99(12):4674-4682. doi:10.1210/jc.2013-4332
- Paramasivam SS, Chinna K, Singh AKK, et. Continuous glucose monitoring results in lower HbA1c in Malaysian women with insulin-treated gestational diabetes: a randomized controlled trial. Diabet Med 2018;35:1118–1129. Pmid:29663517
- Alfadhli E, Osman E, Basri T. Use of a real time continuous glucose monitoring system as an educational tool for patients with gestational diabetes. Diabetol Metab Syndr. (2016) 8:48. 10.1186/s13098-016-0161-5
- Moy FM, Ray A, Buckley BS, West HM. Techniques of monitoring blood glucose during pregnancy for women with preexisting diabetes. Cochrane Database of Systematic Reviews. 2017. doi:10.1002/14651858.cd009613.pub3
- Beck RW, Riddlesworth T, Ruedy K, et al. Effect of Continuous Glucose Monitoring on Glycemic Control in Adults With Type 1 Diabetes Using Insulin Injections. Jama. 2017;317(4):371. doi:10.1001/jama.2016.19975
- Betrán AP, Ye J, Moller A-B, Zhang J, Gülmezoglu AM, Torloni MR. The Increasing Trend in Caesarean Section Rates: Global, Regional and National Estimates: 1990-2014. Plos One. 2016;11(2). doi:10.1371/journal.pone.0148343
- Weir CB, Jan A. BMI Classification Percentile And Cut Off Points. StatPearls. https://www.ncbi.nlm.nih.gov/pubmed/31082114. Published 2019. Accessed May 20, 2020.
- HAPO Study Cooperative Research Group. Hyperglycaemia and Adverse Pregnancy Outcome (HAPO) Study: associations with maternal body mass index. BJOG: An International Journal of Obstetrics & Gynaecology. 2010;117(5):575-584. doi:10.1111/j.1471-0528.2009.02486.x
- Trojner-Bregar A, Blickstein I, Lucovnik M, Steblovnik L, Verdenik I, Tul N. The relationship between cesarean section rate in term singleton pregnancies, maternal weight, and weight gain during pregnancy. Journal of Perinatal Medicine. 2016;44(4). doi:10.1515/jpm-2015-0117
- Rodbard D. Continuous Glucose Monitoring: A Review of Successes, Challenges, and Opportunities. Diabetes Technology & Therapeutics. 2016;18(S2). doi:10.1089/dia.2015.0417
- Mack LR, Tomich PG. Gestational Diabetes. Obstetrics and Gynecology Clinics of North America. 2017;44(2):207-217. doi:10.1016/j.ogc.2017.02.002
- Brown J, Alwan NA, West J, et al. Lifestyle interventions for the treatment of women with gestational diabetes. Cochrane Database of Systematic Reviews. 2017. doi:10.1002/14651858.cd011970.pub2
- Tundidor D, Meek CL, Yamamoto J, Martínez-Bru C, Gich I, Feig DS, Murphy HR, Corcoy R; CONCEPTT Collaborative Group. Continuous Glucose Monitoring Time-in-Range and HbA1c Targets in Pregnant Women with Type 1 Diabetes. Diabetes Technol Ther. 2021 May 25. doi: 10.1089/dia.2021.0073. Epub ahead of print. PMID: 33945304.
Article information:
Published Online: November 2nd, 2021.
IRB Approval: No IRB approval necessary.
Conflict of Interest Declaration: No conflicts of interest to disclose.
Funding Source/Disclosure: No support provided.