Joshua Bezecny1, Rajan Kulkarni, MD, PhD2, Matthew Hiram Taylor, MD 2, Elizabeth Berry, MD2
PNWMSRJ. Published online November 2nd, 2021.Abstract:
Introduction and Objective: Erythema nodosum associated with nivolumab therapy is a rare immune-related adverse event. In four previously reported cases, patients had presented with multiple, painful subcutaneous nodules along the lower extremities following nivolumab therapy.
Conclusion: This report differs from previously described cases and highlights the possibility that erythema nodosum secondary to nivolumab therapy can present clinically with mildly symptomatic subcutaneous nodules discovered incidentally on routine surveillance fluorodeoxyglucose positron emission tomography/computed tomography scanning. These findings can increase the clinician’s concern for malignancy, prompting further work-up, including biopsy and histopathological assessment of the lesions. Our case report can serve to guide the clinician to identify and manage this rare adverse event.
Introduction:
Erythema nodosum (EN) associated with nivolumab therapy is a rare immune-related adverse event (irAE) that has only been described in four prior reports.[1-4] In this case report, we describe a 36-year-old woman with metastatic melanoma on nivolumab who, on routine surveillance fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT), was found to have multiple FDG-avid nodules on her bilateral lower extremities, which led to the discovery of a mildly tender left shin subcutaneous nodule with histopathological findings consistent with EN.
Case Presentation:
A 36-year-old woman with a history of metastatic melanoma on nivolumab presented to dermatology clinic after routine imaging identified several FDG-avid nodules on her lower legs. Her past medical history was notable for IV drug use and positive hepatitis C antibody without evidence of active viral infection. In 2011, she was diagnosed with a melanoma of her left thigh (Breslow depth unknown) and was treated with wide local excision and sentinel lymph node biopsy (negative for lymph node involvement).
In Spring 2019 she was seen by dermatology, and examination of the left shin revealed a 1 centimeter, solitary, firm but mobile, subcutaneous nodule without a visible punctum or epidermal change (Figure 1B). There were no palpable nodules on the right shin or between the left first and second metatarsal heads, at sites of prior FDG uptake. She reported no preceding illness or infections but noted that she had been diagnosed with hepatitis C in the past (without evidence of current infection). The patient denied new medications exposures prior to the appearance of the nodules. She was not aware of any family history of autoimmune disease. A punch biopsy of the left shin nodule revealed septal granulomatous mixed panniculitis with eosinophils, neutrophils, and plasma cells (Figures 2A and 2B), consistent with EN. There was no evidence of vasculitis. Gram, Grocott methenamine silver (GMS) and Fite stains for bacterial, fungal, and atypical mycobacterial organisms (respectively) were negative. There was no polarizable foreign material identified. PET/CT performed February 2019 did not reveal enlarged hilar or mediastinal lymph nodes. She continued nivolumab. As she was not symptomatic, she declined treatment for EN. Careful monitoring for development of sarcoidosis on PET/CT imaging was recommended, as EN has been reported as antecedent to development of sarcoidosis.[1]
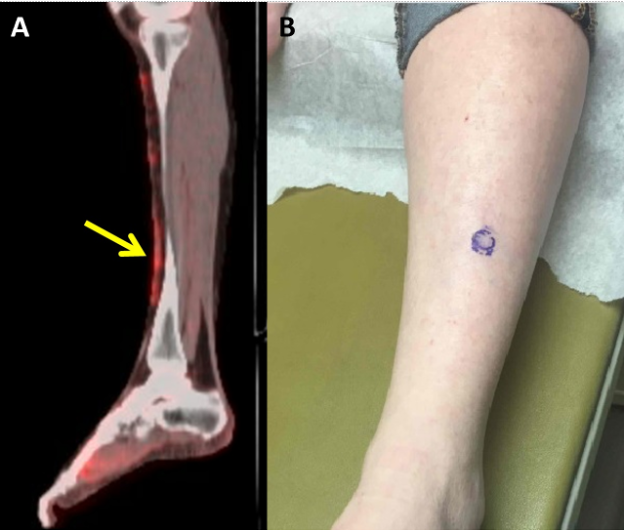
Figure 1. A, FDG PET/CT showing dermal-based soft tissue thickening involving the anterior lower legs with associated hypermetabolic activity, and hypermetabolic focus between first and second metatarsal head. B, Left shin with a 1cm, solitary, firm but mobile subcutaneous nodule without epidermal change.
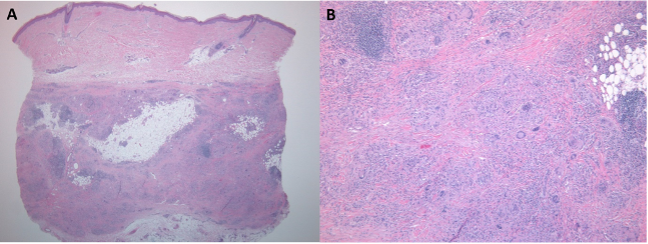
Figure 2. A, Punch biopsy showing septal panniculitis. B, Mixed inflammatory infiltrate with multinucleated giant cells in the septae.
Discussion:
Immune checkpoint inhibitors (ICIs) have dramatically improved the prognosis for individuals with advanced melanoma (including ipilimumab, nivolumab, and pembrolizumab to date).[5] Nivolumab is a human immunoglobulin G4 (IgG4) monoclonal antibody that binds to programmed cell death protein 1 (PD-1), inhibiting its interaction with programmed death-ligands 1 and 2 (PD-L1 and PD-L2), thereby helping to reverse T cell exhaustion and facilitate T cell activation. Nivolumab is FDA-approved for treatment of unresectable stage III and stage IV melanoma as well as adjuvant treatment for stage III melanoma at high risk for relapse.[6,7] By potentiating T cell activity, nivolumab can also lead to a wide array of deleterious immune-related adverse events (irAEs), most commonly rash or pruritis.[8]
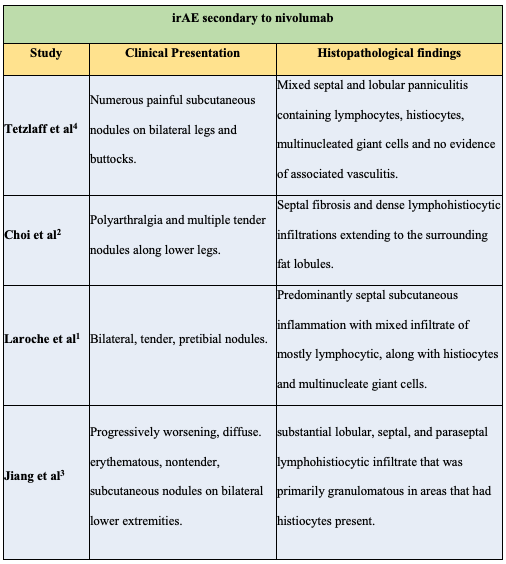
There have been four patients previously described with histopathologic findings compatible with EN related to single agent nivolumab (Table 1).[1-4] EN classically presents with tender, erythematous, subcutaneous nodules and plaques along bilateral lower extremities and occasionally the thighs, arms, trunk, neck and face.[9] FDG, a radioactive tracer, concentrates in areas of increased metabolic activity (e.g. cancer cells) on PET/CT, and will also concentrate in EN nodules due to their increased levels of inflammation. Histologically, EN demonstrates a mixed cellular infiltrate of histiocytes, lymphocytes, giant cells, and occasional eosinophils involving the septae of the subcutaneous fat without evidence of vasculitis.[9] Described as a type IV delayed hypersensitivity reaction, EN can have a wide array of etiologies including infection (predominantly streptococcal pharyngitis, 28-48%), sarcoidosis (11-25%), drugs (3-10%), pregnancy (2-5%), and inflammatory bowel disease (1-4%).[10,11] Up to 55% of cases are idiopathic.11 Hepatitis C has also been reported as a rare cause of erythema nodosum.[12] While our patient did have a history of a positive hepatitis C antibody, she did not have evidence of active infection, making hepatitis C an unlikely etiology for her EN.Table 1: Comparison of previously reported cases of EN secondary to nivolumab.
Conclusion:
In contrast to the previously reported patients, multiple FDG-avid nodules were discovered incidentally on routine surveillance PET/CT on our patient prior to any clinical symptoms presenting. Her symptoms were considerably mild in comparison to prior reports, as her only clinical finding consisted of one mildly tender, subcutaneous nodule along her left shin. Despite her subtle presentation, biopsy results revealed significant septal inflammation, which led to the diagnosis of erythema nodosum, an irAE secondary to nivolumab toxicity. As use of nivolumab continues to grow, routine surveillance PET/CT scanning for disease recurrence may reveal new FDG-avid nodules in an increasing number of asymptomatic or mildly symptomatic patients, which can increase the clinician’s concern for malignancy, prompting further work-up including biopsy and histopathological assessment of the lesions. The addition of this case to the growing body of literature describing irAEs can further guide clinicians to avoid misdiagnosis and to correctly identify subtle presentations of EN-like panniculitis as an irAE of anti-PD-1 therapy.
Learning Points:
- In contrast to previous reports, multiple FDG-avid nodules were discovered incidentally on routine surveillance PET/CT on our patient prior to any clinical symptoms presenting.
- Despite her subtle presentation, biopsy results revealed significant septal inflammation, which led to the diagnosis of erythema nodosum, an irAE secondary to nivolumab toxicity.
- As use of nivolumab continues to grow, routine surveillance PET/CT scanning for disease recurrence may reveal new FDG-avid nodules in an increasing number of asymptomatic or mildly symptomatic patients, which can increase the clinician’s concern for malignancy, prompting further work-up including biopsy and histopathological assessment of the lesions.
Acknowledgements:
I would like to acknowledge and thank doctors Elizabeth Berry, Rajan Kulkarni and Matthew Taylor for sharing this patient’s case with me and for their incredible mentorship.
References
- Laroche A, Alarcon Chinchilla E, Bourgeault E, Doré M-A. Erythema Nodosum as the Initial Presentation of Nivolumab-Induced Sarcoidosis-Like Reaction. J Cutan Med Surg. 2018;22(6):627-629. doi:10.1177/1203475418776934
- Choi ME, Lee KH, Won CH, et al. A case of erythema nodosum-like panniculitis induced by nivolumab in a patient with oesophageal cancer. Australas J Dermatol. 2019;60(2):154-156. doi:10.1111/ajd.12970
- Jiang B, Patino MM, Gross AJ, et al. Diffuse granulomatous panniculitis associated with anti PD-1 antibody therapy. JAAD Case Rep. 2018;4(1):13-16. doi:10.1016/j.jdcr.2017.06.014
- Tetzlaff MT, Jazaeri AA, Torres-Cabala CA, et al. Erythema nodosum-like panniculitis mimicking disease recurrence: A novel toxicity from immune checkpoint blockade therapy—Report of 2 patients. J Cutan Pathol. 2017;44(12):1080-1086. doi:10.1111/cup.13044
- Hargadon KM, Johnson CE, Williams CJ. Immune checkpoint blockade therapy for cancer: An overview of FDA-approved immune checkpoint inhibitors. Int Immunopharmacol. 2018;62:29-39. doi:10.1016/j.intimp.2018.06.001
- Leven C, Padelli M, Carré J-L, Bellissant E, Misery L. Immune Checkpoint Inhibitors in Melanoma: A Review of Pharmacokinetics and Exposure–Response Relationships. Clin Pharmacokinet. June 2019. doi:10.1007/s40262-019-00789-7
- Melanoma Treatment. National Cancer Institute. https://www.cancer.gov/types/skin/hp/melanoma-treatment-pdq. Published June 29, 2019. Accessed on July 20, 2019.
- Marin-Acevedo JA, Chirila RM, Dronca RS. Immune Checkpoint Inhibitor Toxicities. Mayo Clin Proc. 2019;94(7):1321-1329. doi:10.1016/j.mayocp.2019.03.012
- Thurber S, Kohler S. Histopathologic spectrum of erythema nodosum. J Cutan Pathol. 2006;33(1):18-26. doi:10.1111/j.0303-6987.2006.00402.x
- Chowaniec M, Starba A, Wiland P. Erythema nodosum – review of the literature. Reumatologia. 2016;54(2):79-82. doi:10.5114/reum.2016.60217
- Schwartz RA, Nervi SJ. Erythema nodosum: a sign of systemic disease. Am Fam Physician. 2007;75(5):695-700.
- Hadziyannis SJ. Skin diseases associated with hepatitis C virus infection. J Eur Acad Dermatol Venereol. 1998;10(1):12-21. doi: https://doi.org/10.1111/j.1468-3083.1998.tb00922.x
Article information:
Published Online: November 2nd, 2021.
IRB Approval: Not human subjects research, effective September 4, 2019.
Conflict of Interest Declaration:
Joshua Bezecny has no conflicts of interest to declare.
Rajan Kulkarni has no conflicts of interest to declare.
Matthew Hiram Taylor: has received honoraria for participation in advisory boards for Bristol Myers Squibb, Eisai Inc, Novartis, Array Biopharma, LOXO oncology, Bayer, Blueprint Medicines, Arqule, and Sanofi/Genzyme. He has received honoraria for non-promotional/unbranded speaker bureau talks from Bristol Myers Squibb and Eisai Inc.
Elizabeth Gates Berry has served on an advisory board for Bristol Myers Squibb for which she received consultation fees and travel expenses.
Funding Source/Disclosure: We did not receive any financial or other sources of support.