Holly Ryan1, Chuck Chan1, Chaya Prasad MD, MBA1
PNWMSRJ. Published online November 2nd, 2021.Abstract:
Introduction: Autoimmune diseases have been increasing in incidence in the Western world over the last few decades at a faster rate than expected through genetic variation alone. This is suggestive of a correlation between the worsening American diet and inflammatory mediated autoimmunity. Several studies have proposed mechanisms for this correlation including the relationship between the gut microbiome and autoimmune disease, as well as the role of diet composition and the activation of the immune system. Our case report highlights the proposed relationship between dietary choices and clinical severity of Hashimoto’s Thyroiditis (HT).
Case Presentation: A 54-year-old patient with past medical history of HT and leaky gut syndrome presented to her primary care physician for extreme fatigue, rosacea, facial edema, and irregular bowel movements. At the time of presentation, the patient had baseline laboratory tests drawn including lipid panel, thyroid panel, thyroperoxidase (TPO) antibody level, and inflammatory markers. Following six months of diet modification, the patient reported clinical resolution of her symptoms with concordant improvement of her lab values at her six-month evaluation.
Conclusion: Elimination of gluten, dairy, and red meat with the increased consumption of fiber and omega 3 fatty acids was correlated with improved laboratory values from this patient’s baseline lab values. The patient had a decrease of her inflammatory markers including TPO antibody and hs-CRP, with an increase of cardioprotective fatty acids including omega-3 and hs-omega 3 after six months.
Background:
Autoimmune disease plays a major role in patient wellness and health care, currently affecting more than 8% of the US population.[1] The incidence of autoimmune disease has continued to rise over the last 30 years for unknown reasons, with a stable prevalence of the autoimmune genetic pool.[2] This suggests that the increase in autoimmune incidence has largely been influenced by environmental factors.
Some known environmental factors that increase the incidence of autoimmunity include smoking, UV light exposure, infections, and, most importantly, diet.[3,4]
Case Presentation:
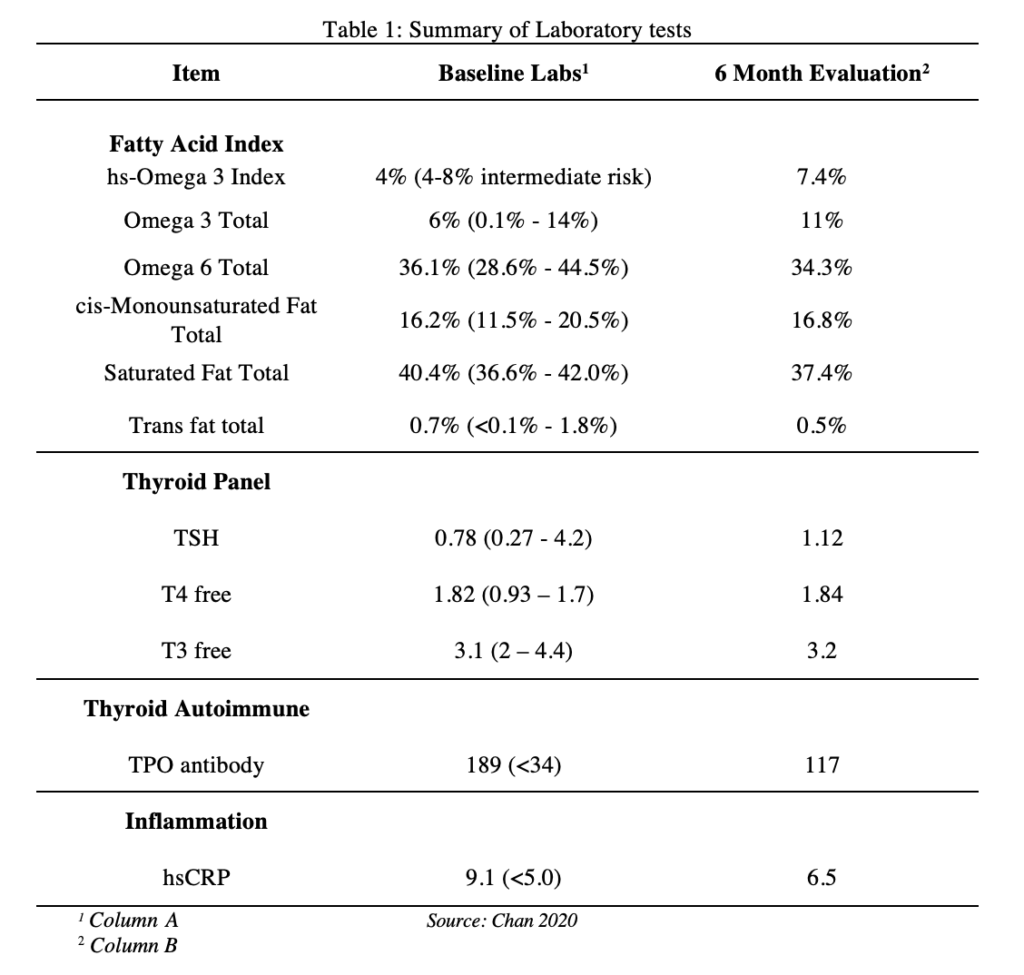
We present a 54-year-old moderately obese female with a past medical history of HT and leaky gut syndrome presented in January 2019 complaining of extreme fatigue, rosacea, facial edema, diarrhea and constipation, bilateral knee pain, excessive hair loss, and weight gain of 25lb. She was diagnosed with HT in 2018 and was prescribed levothyroxine 50 mcg daily. Her diet was high in carbohydrates, animal fat and protein, and high salt intake in the form of fast food, and her symptoms worsened when eating wheat products, refined sugars, and red meat. Pertinent social history included the patient admitting to high stress having to take care of her autistic child. Past medical history and family history was negative for autoimmune disease. Physical exam included rosacea and facial edema with no other significant findings. Her baseline labs included fatty acids, thyroid panel, TPO antibody, and hsCRP (column A, Table 1). She was started on a dietary plan including the reduction of high salt and high fat intake, reduction of omega 6 rich foods and the elimination of dairy products, gluten, red meat, wheat, glyphosate. The patient was also advised to increase consumption of omega 3 rich foods such as avocado and salmon. Fiber intake was increased to 5 cups per day in the form of lentils, beans, nuts, and seeds. She was assigned a personal trainer to manage her weight as well.
Table 1: Summary of Laboratory tests
Discussion:
This patient with a past medical history of HT presented with clinical symptoms of hypothyroidism including fatigue, rosacea, facial edema, alternating diarrhea and constipation, weight gain, bilateral knee arthralgias, and hair loss. These symptoms persisted despite original treatment with levothyroxine 50mcg daily and TSH within normal range.[12] By eliminating dairy products, gluten, red meat, wheat, and glyphosate she reported clinical improvements in weight loss of 37lb, reduction of facial edema, and decreased rosacea in a 6-month span. The patient also demonstrated marked improvement from her baseline labs specifically in TPO antibody levels (189 to 117), hsCRP (9.1 to 6.5), and fatty acid levels (hs omega 3 4.0% to 7.4%).
Several mechanisms connecting diet with autoimmune disease have been proposed15. Gut microbiota has been a major area of research in recent years in the pathogenesis and progression of autoimmune disease. Dysbiosis in HT is found in the form of molecular mimicry and the distribution of epitopes to activate the immune system and induce the loss of self-tolerance. Morphological changes in enterocytes of the duodenum have been observed in patients with HT including increased thickness and increased space between adjacent microvilli. These microscopic changes are consistent with increased intestinal permeability leading to a leaky gut syndrome. The proposed loss of self-tolerance and loss of protective barrier caused by dysbiosis is a major trigger that could contribute to the development of HT.[8,13] Another possible mechanism involves a high fat diet modulating autoimmune disease. Increased fatty acids induces Th17 responses resulting in increased levels of IL-17, a pro-inflammatory cytokine linked to autoimmunity. In addition, a high salt diet intake causes immune activation at the level of T cells which also increases the number of Th17 population and ultimately the upregulation of proinflammatory cytokines.[15] Limitations of our case presentation include the inability to isolate factors that contributed to the improvement of her clinical symptoms and her reduction of inflammation markers. Our patient was started on both a dietary and an exercise intervention simultaneously, which implies that her reduction in inflammation is likely multifactorial. The persistence in her bilateral knee pain suggests a lack of association with a systemic inflammatory process. However, it is also possible that the pain improved passed the 6-month span.
Genetic testing was not performed on our patient, and as a result we do not have any information regarding the possible genetic predisposition such as HLA haplotype. Furthermore, the patient did not have a repeat lipid panel to compare to her baseline labs. Trending the lipid panel could have either supported or rejected the theory that lipid regulation played a role in reducing the severity of the patient’s autoimmune disease.
Learning Points:
- Elimination of gluten, dairy, red meat, and other known inflammatory foods along with increased consumption of fiber and omega 3 fatty acids led to a reported improvement in rosacea and fatigue, and an improvement in TPO antibody levels and hs-CRP from baseline in this patient.
- An increase in this patient’s hs-omega 3 index and omega 3 levels from baseline suggests that the mechanism of reduced systemic inflammation could be due to the protective effects of PUFAs against proinflammatory markers.
Acknowledgements:
Special thanks to Dr. Jennifer Loomis (family practitioner, Loomis Healthy Living in Los Alamitos, CA) for giving us permission and providing us with information on her patient for this case report.
References
- Cormack T. The role of genetics and environmental factors on autoimmune disease incidence with a focus on gender bias in a family case study. Published online April 29, 2019.
- Lerner A, Jeremias P, Matthias T. The world incidence and prevalence of autoimmune diseases is increasing. Int j celiac dis. 2016;3(4):151-155.
- Cruz-Tapias P, Castiblanco J, Anaya J-M. HLA Association with Autoimmune Diseases. El Rosario University Press; 2013.
- Marder W, Vinet É, Somers EC. Rheumatic autoimmune diseases in women and midlife health. Womens Midlife Health. 2015;1. doi:10.1186/s40695-015-0012-9
- Smidowicz A, Regula J. Effect of nutritional status and dietary patterns on human serum C-reactive protein and interleukin-6 concentrations. Adv Nutr. 2015;6(6):738-747.
- Niland B, Cash BD. Health benefits and adverse effects of a gluten-free diet in non-celiac disease patients. Gastroenterol Hepatol (N Y). 2018;14(2):82-91.
- Vazquez-Roque MI, Camilleri M, Smyrk T, et al. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: effects on bowel frequency and intestinal function. Gastroenterology. 2013;144(5):903-911.e3.
- Virili C, Fallahi P, Antonelli A, Benvenga S, Centanni M. Gut microbiota and Hashimoto’s thyroiditis. Rev Endocr Metab Disord. 2018;19(4):293-300.
- Mincer DL, Jialal I. Hashimoto Thyroiditis. In: StatPearls. StatPearls Publishing; 2020.
- Abbott RD, Sadowski A, Alt AG. Efficacy of the Autoimmune Protocol diet as part of a multi-disciplinary, supported lifestyle intervention for Hashimoto’s thyroiditis. Cureus. 2019;11(4):e4556.
- Figueroa-Vega N, Alfonso-Pérez M, Benedicto I, Sánchez-Madrid F, González-Amaro R, Marazuela M. Increased circulating pro-inflammatory cytokines and Th17 lymphocytes in Hashimoto’s thyroiditis. J Clin Endocrinol Metab. 2010;95(2):953-962.
- Garber JR, Cobin RH, Gharib H, et al. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18(6):988-1028.
- Mori K, Nakagawa Y, Ozaki H. Does the gut microbiota trigger Hashimoto’s thyroiditis? Discov Med. 2012;14(78):321-326.
- Nielsen CH, Brix TH, Leslie RGQ, Hegedüs L. A role for autoantibodies in enhancement of pro-inflammatory cytokine responses to a self-antigen, thyroid peroxidase. Clin Immunol. 2009;133(2):218-227.
- Ferrucci L, Cherubini A, Bandinelli S, et al. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J Clin Endocrinol Metab. 2006;91(2):439-446.
Article information:
Published Online: November 2nd, 2021.
IRB Approval: The methods used in this study were approved by the Western University of Health Sciences Institutional Review Board (reference#: 19/RFD/038)
Conflict of Interest Declaration: The authors of this case report have no conflicts of interest to disclose.
Funding Source/Disclosure: No support was provided for this research.